Research
Our Research-Nuclear pore complex (NPC)
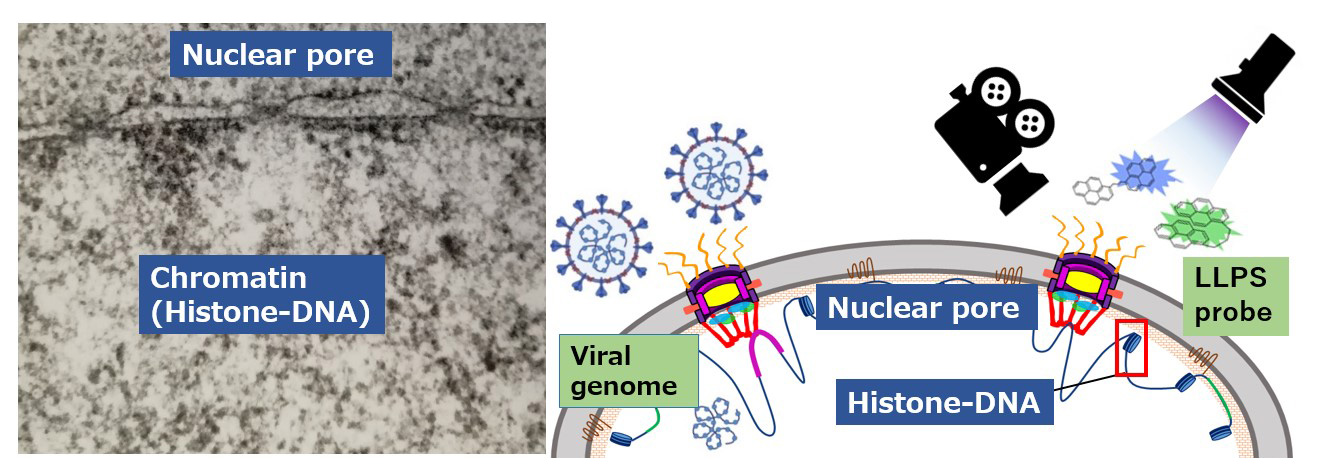
1) NPC peripheral structural dynamics
2) NPC and its related diseases
3) Virus invasion routes to NPC
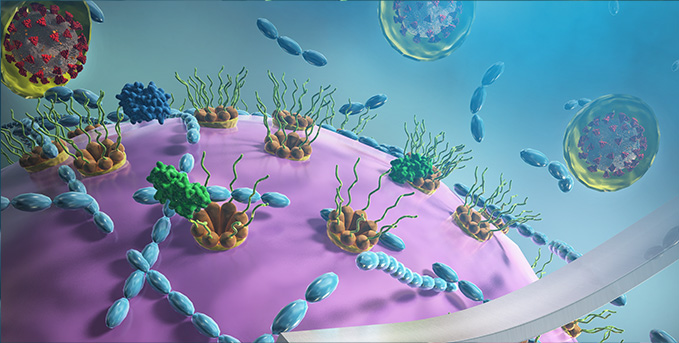
What did we discover?

I set up a lab in Kanazawa University to investigate the Nuclear pore complex (NPC) in 2008.
After a decade investigating, our laboratory explored additional functions of NPCs beyond nuclear transport and found that nucleoporins Rae1, Tpr, Nup358, Nup62, Nup58, and Nup88 play critical roles in maintaining spindle bipolarity, centrosome and mid-body homeostasis during cell division. For instance, we provided several lines of evidence that the phosphorylation of the cohesin subunit, SMC1, stimulates binding to mitotic Rae1, a phenomenon recently confirmed by others to form part of a mechanism of antimitotic catastrophe in early tumorigenesis.
We found that nuclear pore protein Nup62 trafficking transcription factor p63 and importins (KPNA4) oncogenic signaling during nuclear pore trafficking. In 2012, we first showed that the nuclear pore protein, Tpr, involved in autophagy, which affects ependymoma in humans. Using high-speed atomic force microscopy (HS-AFM), we recently showed that FG-Nups are in a liquid–liquid phase separated (LLPS) state in which rapid and transient intermediates of FG filaments and sometimes co-exist with a central plug during conformational changes in cancer cells and organoids. The real-time HS-AFM images also suggested that the NPC central channel has a moist cobweb-like structure.
We are now interested in how different Nups proteins interact with the genome and perform important roles in regulation of gene expression; and how different types of viruses are transported into the cell and hijacking the nuclear pores.
Research interview
"Bio-AFM
Frontier Research Center”, T. Uchihashi T. Ando
R. Wong T. Fukuma
Impact pp. 23-25, 2017
